
Does CBD Treat Depression?
Cannabidiol – often abbreviated as CBD – is a truly remarkable, powerful molecule of the cannabis plant that has dozens of potential health benefits in
CBD occupies a unique economic space; it’s a relatively new industry (hemp was only legalized for nationwide commercial production in 2018) without a robust regulatory infrastructure behind it.
Given the regulatory limbo, analysts have dubbed the budding CBD industry a “Wild West” in the sense that there is no single established authority governing its production, marketing, and sale.
The lax state of CBD regulation contrasts with, for example, the conventional pharmaceutical industry, which relies on strict FDA guidelines to screen new products for safety and efficacy.
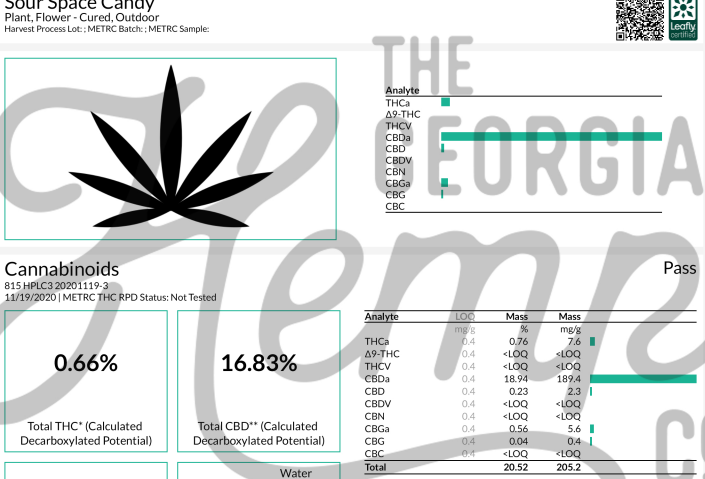
As a result, third-party laboratories have stepped in to fill the gap by scientifically analyzing and then endorsing passing products with a seal of approval, termed in the industry certification of analysis (COA).
Let’s get into what COAs say, how to read them, and why they’re important to protect consumer safety.
Certificates of analysis, issued by accredited independent third-party labs, contain detailed quantitative data regarding the chemical makeup of a given substance – in the CBD context, of a product containing cannabidiol.
Their purpose is to guarantee the consumer that the CBD product they are using is safe and effective by providing an exhaustive list of its content.
If you’re familiar with the old adage “you can’t let the cops investigate themselves,” you’ll understand the dilemma that CBD producers face – without FDA endorsement (more on that issue later), how to assure consumers they are getting a product that contains all the advertised beneficial components and none of the toxins or unnecessary added ingredients sometimes discovered in supplements?
As a wise consumer, to protect your health and safety, you need confirmation of the product’s quality from a disinterested outside expert with no stake, financial or otherwise, in the product’s success or failure.
That’s why it’s important for CBD manufacturers to obtain independent, third-party verification of the purity of their products to assuage consumers’ concerns.
A COA for a CBD product should indicate statistical levels of all of the following data:
The research into which pesticides are safe for use on CBD crops is minimal as is the regulatory scheme, given that the cannabis plant was only until very recently entirely illegal at the federal level.
Purdue pharmacist Janna Beckerman explains:
“When a highly valued crop has problems, people are going to apply pesticides. But unlike many of our currently regulated crops, cannabis can be dried or turned into oils, concentrating it and any chemicals put onto the plants. We don’t know how those concentrations might affect users who ingest and inhale the end products.”
In addition to providing information related to pesticide residue, certifying labs also inspect CBD products for potentially deadly solvents used in the curation and manufacturing processes:
“In many cases, the solvents used during the manufacturing process are harmful or toxic. Naptha and petroleum ether have boiling points ranging from 30°C to 200°C. These solvents are harmful and flammable, and some solvents, such as hexane and benzene are, considered neurotoxic.”

As well as screening CBD products for toxic chemicals like solvents or pesticides, COAs guarantee the consumer that they are getting the CBD:THC ratio they are looking for.
This distinction is particularly important in the context of CBD isolate vs. full-spectrum CBD:
For more information on the differences between CBD and THC, and between CBD isolate and full-spectrum CBD and their respective benefits, check out our article on that topic.

Consider a recent study titled Labeling Accuracy of Cannabidiol Extracts Sold Online published in the Journal of the American Medical Association:
“Among CBD products purchased online, a wide range of CBD concentrations was found, consistent with the lack of an accepted dose. Of tested products, 26% contained less CBD than labeled, which could negate any potential clinical response… These findings highlight the need for manufacturing and testing standards, and oversight of medicinal cannabis products.”
That means that over a quarter of CBD sold on the web contains less CBD than its manufacturers advertise. As a result, if you’re one of the unlucky millions who have bought these products unwittingly, you might be losing out on the numerous potential health benefits because you’re consuming too little of the compound.
Important: Reputable CBD vendors will publish their COAs online. If you don’t see any or can’t find the page where they’re hosted, ask to see them. If the company won’t provide them or doesn’t have them, that should raise some red flags.
We screen our CBD products for quality ourselves, as well as commission third-party labs to vet them.
Check out our comprehensive vault of COAs for the high-quality Sympleaf CBD products available through our online store.

At Sympleaf, we are committed to transparency. Our partner labs verify that the products we sell contain the advertised CBD content, don’t exceed the legal limits of 0.3% THC concentrations under federal law, and don’t contain harmful toxins or chemicals like pesticides or heavy metals.
No. The FDA, at this point, is limited in its regulatory authority over cannabis products. Labs such as ChemHistory that issue certifications for non-pharmaceutical CBD products act independently, without collaboration with the FDA:
“CBD-containing products do not currently qualify as dietary supplements due to the ‘exclusion clause’ in the Dietary Supplement Health and Education Act (DSHEA) of 1994.”
As a result of CBD’s status in legal purgatory, despite extensive evidence of CBD’s potential health benefits, vendors and natural healthcare providers are barred from claiming that CBD can “diagnosis, cure, mitigate, treat, or prevent a disease.”
The law often struggles to keep up with fast-developing industries. Congress has, in fact, recently considered expanding the FDA’s oversight role in the cannabis industry. Stay tuned for more developments on that front.
We’re always available to discuss CBD certification of analysis and other regulatory mechanisms to ensure high quality of products. To get in touch, contact Sympleaf.
Stay informed; knowledge is power.

Cannabidiol – often abbreviated as CBD – is a truly remarkable, powerful molecule of the cannabis plant that has dozens of potential health benefits in

Alzheimer’s disease (AD) – along with a host of other neurodegenerative conditions that impact brain health – is nothing short of a public health catastrophe

To date, CBD (“cannabidiol” in long-form) is the single most potent cannabis compound that researchers have isolated to research its potential health benefits. Here, we
Made in Atlanta. © FOOD AND DRUG ADMINISTRATION (FDA) DISCLOSURE These statements have not been evaluated by the Food and Drug Administration. These products are not intended to diagnose, treat, cure, or prevent any disease.